Rightway FACTS™ —
Factory Audit Checklist (Free)
Built on decades of hands-on audits across Asia’s supply chains.

What is a factory audit and why does it matter?
A factory audit is a structured, on-site assessment of a manufacturer’s systems, capacity, workplace environment, and capabilities against your requirements or recognized standards. It goes beyond checking a single batch of goods—it evaluates whether the factory can reliably meet your quality, compliance, and delivery needs over time. Independent bodies describe audits as tailored programs that verify a supplier can fulfill an order to specification and market/regulatory requirements, not just today but repeatedly.
For startups, audits serve two high-leverage moments: (1) final supplier qualification before placing first POs, and (2) ongoing supplier management to ensure capabilities don’t drift as you scale. This reduces launch delays, certification surprises, and expensive firefighting. (Rightway’s FACTS™ — Factory Audit Framework operationalizes this with 0–5 scoring and must-pass gates.)
What does a factory audit cover?
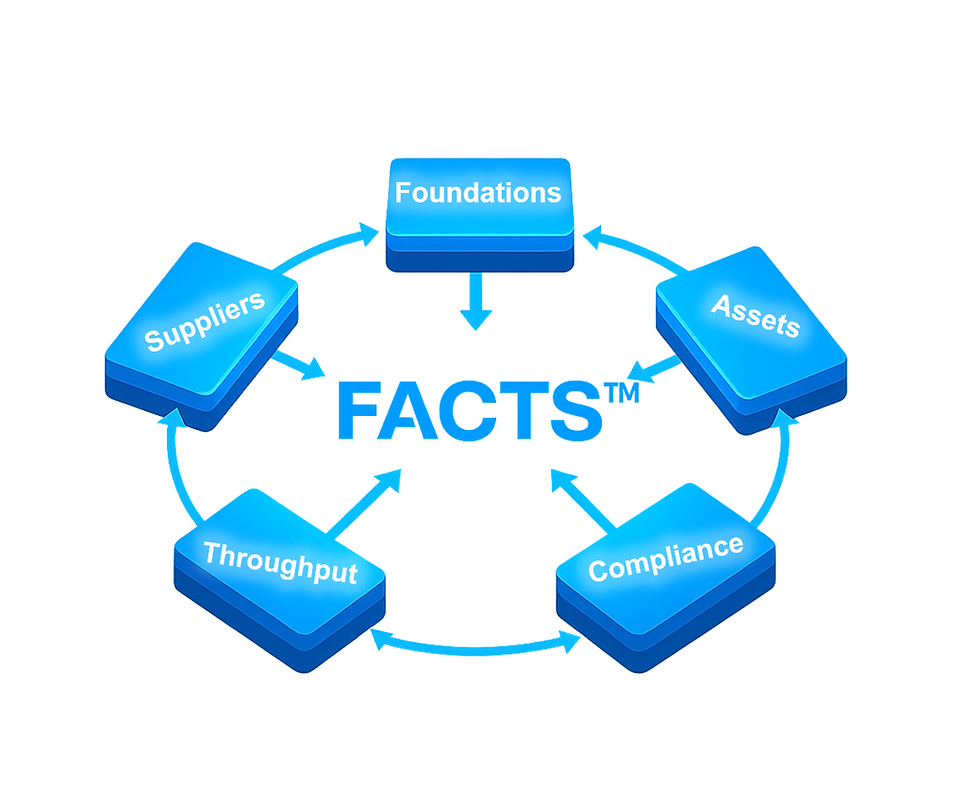
(5 pillars of Rightway FACTS™)
A factory audit should look beyond a single batch of parts. It must confirm that the system behind your product can deliver quality, compliance, and capacity—repeatably. The Rightway FACTS™ — Factory Audit Framework turns that requirement into five practical pillars you can score (0–5) and act on with must-pass gates. Use this section as a factory audit checklist and a roadmap for CAPA (Corrective and Preventive Action).
FACTS stands for:
-
Foundations
-
Assets
-
Compliance & NPI
-
Throughput
-
Suppliers
1) Foundations — Quality System & Governance
Goal: Prove the factory is managed to a standard and can sustain improvements as you scale.
What we verify
-
Quality system certification (ISO 9001 / ISO 14001 / IATF 16949 or equivalent) and the scope that actually covers your product line.
-
Change control for design/process (engineering change, customer notification, document updates, prove-out plans).
-
Document control & training: current work instructions in the operator’s language; training records matched to roles; internal audits with no repeat findings.
-
Problem solving & escalation: 8D/CAPA discipline, closure lead-time, lessons learned deployment across lines/sites.
Evidence we look for
-
Valid certificates, organization chart with named owners, change logs, audit schedules, 8D/CAPA dashboard, training matrices.
Must-pass gates
-
Certified QMS (Quality Management System) in scope of production ≥3/5
-
Formal change management (with customer approval where required) ≥3/5
Startup tip: At EVT, accept “3” with a dated plan; by PVT/MP, expect “4–5”.
2) Assets — Capacity & Technical Capability
Goal: Ensure the people, equipment, tooling, and labs exist to hit your volume and quality targets.
What we verify
-
Like-for-like experience at comparable volumes; run-at-rate history for similar parts.
-
Tooling & fixturing: availability (in-house or strategic partner), preventive maintenance, spare strategy.
-
Metrology & test: equipment and staff to run DV/PV, functional tests, reliability screens.
-
Space & major equipment: installed capacity vs. your ramp (no hidden capex bottlenecks).
Evidence we look for
-
Capacity model, equipment list, PM logs, run-at-rate results, gauge inventory, lab capability matrix.
Must-pass gates
-
Metrology/test capability for critical checks ≥3/5
Startup tip: Ask for a capacity headroom view (people/equipment) for your next 2–3 volume steps.
3) Compliance & NPI — APQP/PPAP, CE/FCC Readiness (using consumer electronics as an example)
Goal: Map your product to the right process & regulatory controls so launches don’t slip.
What we verify
-
Feasibility before quote (manufacturability, special characteristics, critical suppliers).
-
APQP with phase gates and management tracking (EVT → DVT → PVT → MP).
-
PFMEA linked to Control Plans and process flow; RPN (Risk Priority Number) reduction proof.
-
Regulatory readiness (IMDS/REACH/RoHS/Conflict Minerals; CE/FCC test planning).
Evidence we look for
-
Feasibility minutes, APQP tracker, PFMEA/Control Plan pair, PPAP samples (if applicable), regulatory declarations/test plans.
Must-pass gates
-
Feasibility review completed ≥3/5
-
APQP with gated timing under management review ≥3/5
-
PFMEA ↔ Control Plan linkage ≥3/5
-
Regulatory controls identified ≥3/5
Startup tip: Tie each claim on the datasheet to a test method & owner before DVT.
4) Throughput — Process Control, Traceability & Readiness
Goal: Keep the line stable: right first time, controlled variation, and fast containment if anything drifts.
What we verify
-
Lean flow (layout, pull, standardized work, low WIP) and daily tier meetings with visible KPIs (FTT, OEE, shortages).
-
Measurement system capability: Gage R&R / MSA for critical CTQs.
-
Control Plan execution at the station: correct revision of instructions, setup sign-offs, first-piece inspections.
-
Traceability end-to-end (raw → WIP → finished goods) with off-site backup.
-
Nonconformance control: lockable red bins, isolation/containment, disposition workflow.
Evidence we look for
-
MSA reports, calibration logs, station binders (live), traceability samples, NCR/hold tickets, visual factory boards.
Must-pass gates
-
Gage R&R/ MSA for critical measurements ≥3/5
-
Control Plan at point of use & followed ≥3/5
-
Full lot traceability ≥3/5
Startup tip: Promote audit findings → inspection focus (e.g., if CTQ MSA is weak, tighten in-process sampling on that feature).
5) Suppliers — Incoming Quality & Sub-supplier Control
Goal: Prevent upstream surprises. Your output is only as good as their inputs.
What we verify
-
Sub-supplier selection process and requirement flow-down (prints, specs, special characteristics).
-
Change control upstream (ECR, resourcing, material changes) with customer communication records.
-
Incoming quality: documented sampling/testing, reaction plans, quarantine areas with restricted access.
-
Performance management: on-time, PPM, formal reviews, and corrective programs with key subs.
Evidence we look for
-
Approved vendor lists, PO/quality clauses, sub-supplier PPAP/use of APQP, IQC logs, quarantine procedures.
Must-pass gates
-
Incoming inspection strategy + reaction plan ≥3/5
Startup tip: For any single-source item, require a deviation & containment playbook before ramp.
How does the Rightway FACTS™ work step-by-step?
Pre-audit desk review (documents)
We start by checking key records before setting foot on site: ISO/IATF certificates, org chart, APQP tracker, PFMEA ↔ Control Plans, MSA/Gage R&R, calibration logs, and incoming quality procedures. This makes the audit targeted and efficient.
On-site assessment (line walk, sampling, interviews)
Our auditors verify how things actually run: walking the line, checking Control Plans at stations, sampling CTQs, confirming traceability, and interviewing operators and engineers. It’s about seeing evidence in practice, not just on paper.
Scoring 0–5 & must-pass gates
Each item is scored 0–5. Critical checkpoints (like PFMEA–Control Plan linkage, Gage R&R, and traceability) are must-pass gates—any score under 3/5 means STOP until corrected. This avoids hidden risks slipping into builds.
CAPA plan & re-audit cadence
Findings convert into a corrective plan with owners and due dates. Thresholds rise as you scale: EVT can accept 3/5 with a closure plan, while by PVT/MP all gates must reach 4–5/5. Re-audits verify fixes and feed directly into inspection priorities.
👉 Unlike generic checklists, Rightway FACTS™ adds quantitative scoring and stop criteria — so you know exactly when to proceed and what to fix before it risks your launch. We choose supplier by FACTS, not relationship!
What’s inside the free factory audit checklist?
20-items startup-ready checklist
Use the checklist as a fast, practical factory audit starting point. It focuses on what actually moves risk and lead time for startups. Example items:
-
Certified QMS in scope (ISO 9001 / IATF 16949)
-
Change control for design/process (with customer notification)
-
PFMEA ↔ Control Plan linkage on CTQs
-
Gage R&R / MSA for critical measurements
-
Calibration & gauge list at point of use
-
End-to-end traceability (raw → WIP → FG)
-
Incoming quality & quarantine with reaction plans
-
Sub-supplier change control & communication records
Scoring template (0–5) + gates
-
0–5 scale: 5 best practice; 4 documented & implemented; 3 defined & partially deployed; 2 inconsistent; 1 intent only; 0 absent.
-
Must-pass gates (any <3/5 = STOP): MSA/Gage R&R, Control Plan at station, Traceability, Regulatory controls (e.g., REACH/RoHS/IMDS).
Ready to qualify your next factory the right way?
Choosing a supplier isn’t just about price—it’s about protecting your launch, your brand, and your investors’ trust. With Rightway, you get more than a checklist:
-
Local presence in Asia (Taiwan, China, SEA) means faster scheduling, bilingual engineers, and lower travel costs.
-
Decades of cumulative hands-on experience across 40+ startups ensures you avoid common pitfalls.
-
Integrated audits + inspections close the loop from system assurance to shipment verification.
-
Quantified scoring & must-pass gates give you clarity: when to move forward, when to stop, and what to fix.
👉 Whether you’re preparing for EVT, scaling to MP, or re-checking a critical supplier, Rightway helps you save time, cut risk, and launch with confidence.

FAQ
Request your free factory audit checklist Now
Take the first step to qualifying suppliers the right way!
Fill out the form below to get your free 20-item checklist and start benchmarking factories with the Rightway FACTS™ framework.
This 20-item checklist is a startup-ready subset of the full Rightway FACTS™ framework. It highlights the most common “showstopper” risks, while the full 60+ item Pro version (used internally in projects) dives deeper into daily management, nonconformance control, and category-specific requirements.
